
Recall bias in a case-control study is the increased likelihood that those with the outcome will recall and report exposures compared to those without the outcome. In other words, even if both groups had exactly the same exposures, the participants in the cases group may report the exposure more often than the controls do. Recall bias may lead to concluding that there are associations between exposure and disease that do not, in fact, exist. A case-control study is a good tool for exploring risk factors for rare diseases or when other study types are not feasible. Many times an investigator will hypothesize a list of possible risk factors for a disease process and will then use a case-control study to see if there are any possible associations between the risk factors and the disease process. The investigator can then use the data from the case-control study to focus on a few of the most likely causative factors and develop additional hypotheses or questions.
What’s the difference between a case-control study and a longitudinal study?
Indeed, RCTs typically follow patients for only a small fraction of the time that the drug would be used in clinical practice, especially when the medications are for chronic diseases. An additional use of case-control studies for therapeutic effectiveness is to investigate variables or exposures that can guide clinicians in choosing the preferred neurosurgical treatment when more than one therapeutic option exists. An example in the neurosurgical literature includes the study reported by Lennarson et al27 described above utilizing a nested case-control study that evaluated age as a risk factor for nonfusion with halo-vest immobilization for treatment of type II odontoid fractures (Figure 3). The premise for this study was based on existing class III evidence showing no clear difference between the surgical interventions versus halo-vest immobilization for treatment of these fractures. The results of this study provided strong class II medical evidence that age is a significant risk factor for nonfusion with halo-vest immobilization.27 Based on this finding, age can be used to define a treatment algorithm for choosing surgical intervention versus halo-vest immobilization. Similar case-control study designs could be used for other conditions with apparently equivocal treatment options and the findings could help guide the determination of appropriate neurosurgical treatment in subpopulations of patients.
Frequently asked questions
Association of granulocyte macrophage colony-stimulating factor and interleukin-17 levels with obsessive–compulsive ... - Nature.com
Association of granulocyte macrophage colony-stimulating factor and interleukin-17 levels with obsessive–compulsive ....
Posted: Fri, 03 Nov 2023 07:00:00 GMT [source]
This is important, for the statistical analyses appropriate for supporting the conclusion from such studies, the amount of bias control possible, and the ability to estimates odds and relative risk are different depending on study design. The neurosurgeon hoping to appropriately incorporate the results of the studies she reads into practice needs to have at least a fundamental understanding of these issues related to study design to avoid placing unjustified confidence in the conclusions of a study done with insufficient rigor. A frequent source of controls is patients from the same hospital who do not have the outcome. However, hospitalised patients often do not represent the general population; they are likely to suffer health problems and they have access to the health care system. An alternative may be to enroll community controls, people from the same neighborhoods as the cases. Care must be taken with sampling to ensure that the controls represent a ‘normal’ risk profile.
What Is a Case-Control Study? Definition & Examples
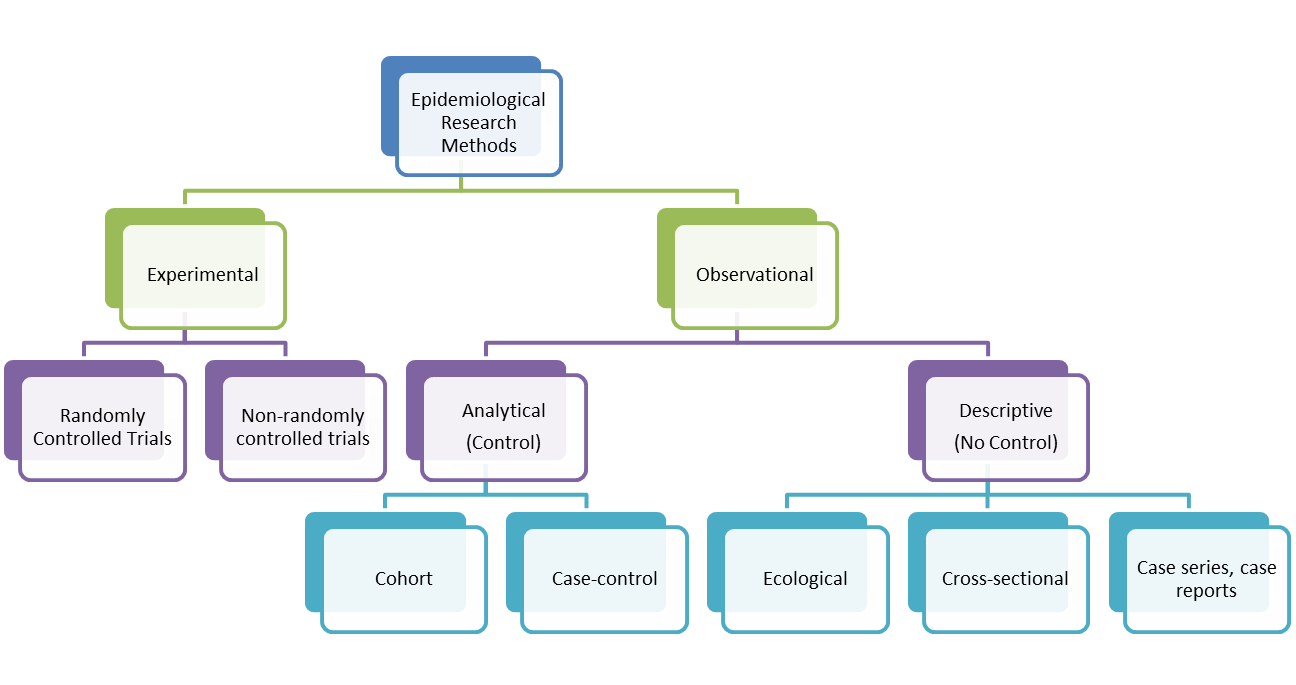
If we sample the study participants based on exposure and move towards the outcome, it is a cohort study. However, if we sample the participants based on the outcome (some with outcome and some do not) and study the exposures in both these groups, it is a case-control study. For cohort studies, the drug–outcome association is usually expressed as a relative risk, a relative rate, or a hazard ratio. Advanced statistical techniques are used to account for factors other than the drug exposure of interest that might distort the drug–outcome association. These factors or potential confounders are often handled simultaneously with multivariable regression models. Because these statistical models account for measured variables, it is crucial that the data source capture as many potential confounding variables (or proxies of confounders) as possible.

A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the ‘case’, and those without it are the ‘control’. Beyond color correction, the panel offers a range of transport and grading controls conveniently positioned around the edges.
Types of Controls
In case-control studies, participants are recruited on the basis of disease status. Thus, some of participants have the outcome of interest (referred to as cases), whereas others do not have the outcome of interest (referred to as controls). Case-control studies are less expensive and quicker to conduct (compared with prospective cohort studies at least).
Risk Factors and Sampling
In cross-sectional studies, researchers are simply examining a group of participants and depicting what already exists in the population. Researchers will first identify the two groups, and then look back in time to investigate which subjects in each group were exposed to the condition. Case control studies are also known as "retrospective studies" and "case-referent studies." An important technique for adding power to a study is to enroll more than one control for every case.
Methodology Series Module 2: Case-control Studies
Then through further exploration, often using other study types (such as cohort studies or randomized clinical studies) the researcher may be able to develop further support for the evidence of the possible association between the exposure and the outcome. The most commonly cited disadvantage in case-control studies is the potential for recall bias.[2] Recall bias in a case-control study is the increased likelihood that those with the outcome will recall and report exposures compared to those without the outcome. The first is to test a preliminary hypothesis to determine if a lengthy and costly RCT is justified. Additionally, this study design may be the only practical option for studying the treatment of a rare disease where enrollment would be insufficient for an RCT, or where randomization would be unethical.
About this article
Moreover, while the estimand may be identifiable, there need not exist an estimator with the desired properties (see e.g. [5]). Here, our focus is on identification, so that the purely statistical issues of the next step in causal inference, estimation, can be momentarily put aside. Thus, the outcome is measured after exposure in retrospective cohort studies, whereas the outcome is measured before the exposure in case-control studies. Case-control studies may prove an association between exposures and outcomes, but they can not demonstrate causation.
In addition, those with the outcome are more likely to recall and report exposures more clearly than those without the outcome. A case-control study looks at people who already have a certain condition (cases) and people who don’t (controls). By comparing these two groups, researchers try to figure out what might have caused the condition.
These additional complexities and designs are beyond the scope of this paper and represent an interesting direction for future research. We establish how, and under which conditions, various causal estimands relating to intention-to-treat or per-protocol effects can be identified based on the data that are collected under popular sampling schemes (case-base, survivor, and risk-set sampling, with or without matching). We present a concise summary of our identification results that link the estimands to the (distribution of the) available data and articulate under which conditions these links hold. Similarly, the researcher must recognize the potential for failing to identify confounding variables or exposures, introducing the possibility of confounding bias, which occurs when a variable that is not being accounted for that has a relationship with both the exposure and outcome. This can cause us to accidentally be studying something we are not accounting for but that may be systematically different between the groups. There are two reasons why, in case-control studies, large samples are desirable, and whymany controls may be matched to a single case.
However, cohort studies with long observation periods may be more susceptible to losses to follow-up and to inaccurate measurement of exposures and outcomes. Large numbers of patients may be required to precisely estimate meaningful drug–outcome associations, especially for rare outcomes or outcomes that take a long time to occur. Observational studies are methodologically difficult, susceptible to bias and confounding, and difficult to interpret, given the many types of bias potentially at play. For these reasons, observational studies are limited to studying drug–outcome associations and cannot be used to measure the causal effects of drugs. Many of these techniques can account for multiple potential confounders simultaneously.

No comments:
Post a Comment